After a week when giant pharma companies such as Moderna, Pfizer, and AstraZeneca announced that their experimental COVID-19 vaccines are effective in preventing the infection, another company filed for FDA’s approval.
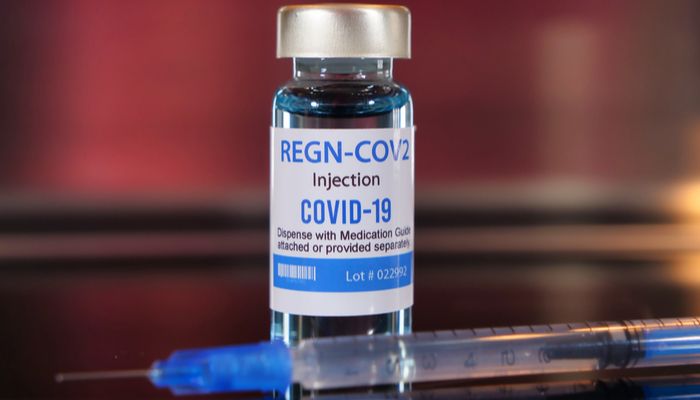
The drug developed by Regeneron has received earlier today the emergency use authorization (EUA) from the Food and Drug Administration. It is the same drug that Trump used when he contracted the virus.
According to studies, Regeneron’s vaccine is effective when administrated shortly after diagnosis. The authorization allows it to be used on patients who are at risk of severe illness.
By the end of November, Regeneron will have doses for roughly 80,000 patients. By the end of January 2021, it will have doses available for 300,000 people.
The number of COVID-19 continues to rise; at the moment of writing, the US has more than 12 million confirmed cases.
After the news, Regeneron stock price went up 3.9%.
Read here about AstraZeneca’s progress!
Sources: bbc.com, Aljazeera.com